
is a Chan Zuckerberg Biohub Investigator. and J.A.T.), NIH Grant R37-AI036929 (to J.A.T.), the Howard Hughes Medical Institute (to J.A.T.), and NSF Grant DMR-1121288 (to D.B.W.). This work was supported by National Institutes of Health (NIH) Director’s New Innovator Awards DP2OD006466 (to K.C.H.) and DP2OD008735 (to D.B.W.), National Science Foundation (NSF) CAREER Award MCB-1149328 (to K.C.H.), the Stanford Systems Biology Center funded by NIH grant P50 GM107615 (to K.C.H. Arjes for feedback, antibodies and strains. Amberg-Johnson for assistance with immunoblotting, and T. The authors thank members of the Huang, Theriot, and Weibel laboratories for discussions, K. Calibration of atomic-force microscope tips. Separation and quantification of muropeptides with high-performance liquid chromatography. The composition of the murein of Escherichia coli.
High-throughput, highly sensitive analyses of bacterial morphogenesis using ultra performance liquid chromatography. Computer control of microscopes using μManager. Role of the rfe gene in the synthesis of the O8 antigen in Escherichia coli K-12. Lysozyme counteracts β-lactam antibiotics by promoting the emergence of L-form bacteria. Distinct single-cell morphological dynamics under beta-lactam antibiotics. De novo morphogenesis in L-forms via geometric control of cell growth. Mechanical genomics identifies diverse modulators of bacterial cell stiffness. Bending forces plastically deform growing bacterial cell walls. Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Chemical conditionality: a genetic strategy to probe organelle assembly.
High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: structural basis for permeability. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. The chemical structure of the lipid A component of lipopolysaccharides from Vibrio cholerae. A pH titration study on the ionic bridging within lipopolysaccharide aggregates. Measuring the stiffness of bacterial cells from growth rates in hydrogels of tunable elasticity. Engineering Tables and Data (Springer Science & Business Media, Berlin, 2012). Die Plasmolytische Studien über die Wand Vacuolen (G. Response of Escherichia coli growth rate to osmotic shock. Biophysical characterization of changes in amounts and activity of Escherichia coli cell and compartment water and turgor pressure in response to osmotic stress. Analysis of surface protein expression reveals the growth pattern of the Gram-negative outer membrane. Supramolecular assemblies underpin turnover of outer membrane proteins in bacteria. Bacterial lipopolysaccharides form physically cross-linked, two-dimensional gels in the presence of divalent cations. Herrmann, M., Schneck, E., Gutsmann, T., Brandenburg, K. Biophysics of bacterial walls viewed as stress-bearing fabric. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Quantitative bausteinanalyse der stützmembran in der zellwand von Escherichia coli B.
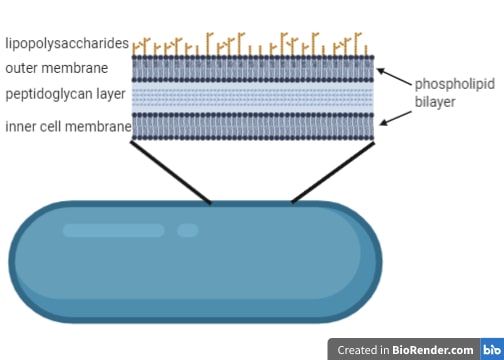
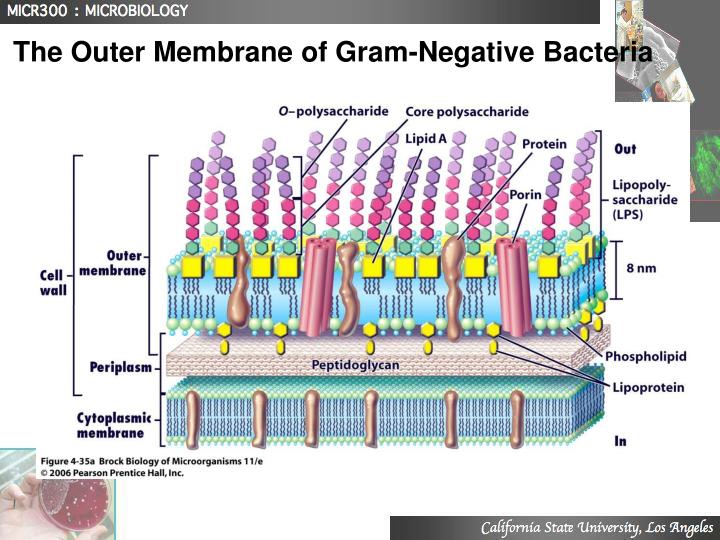
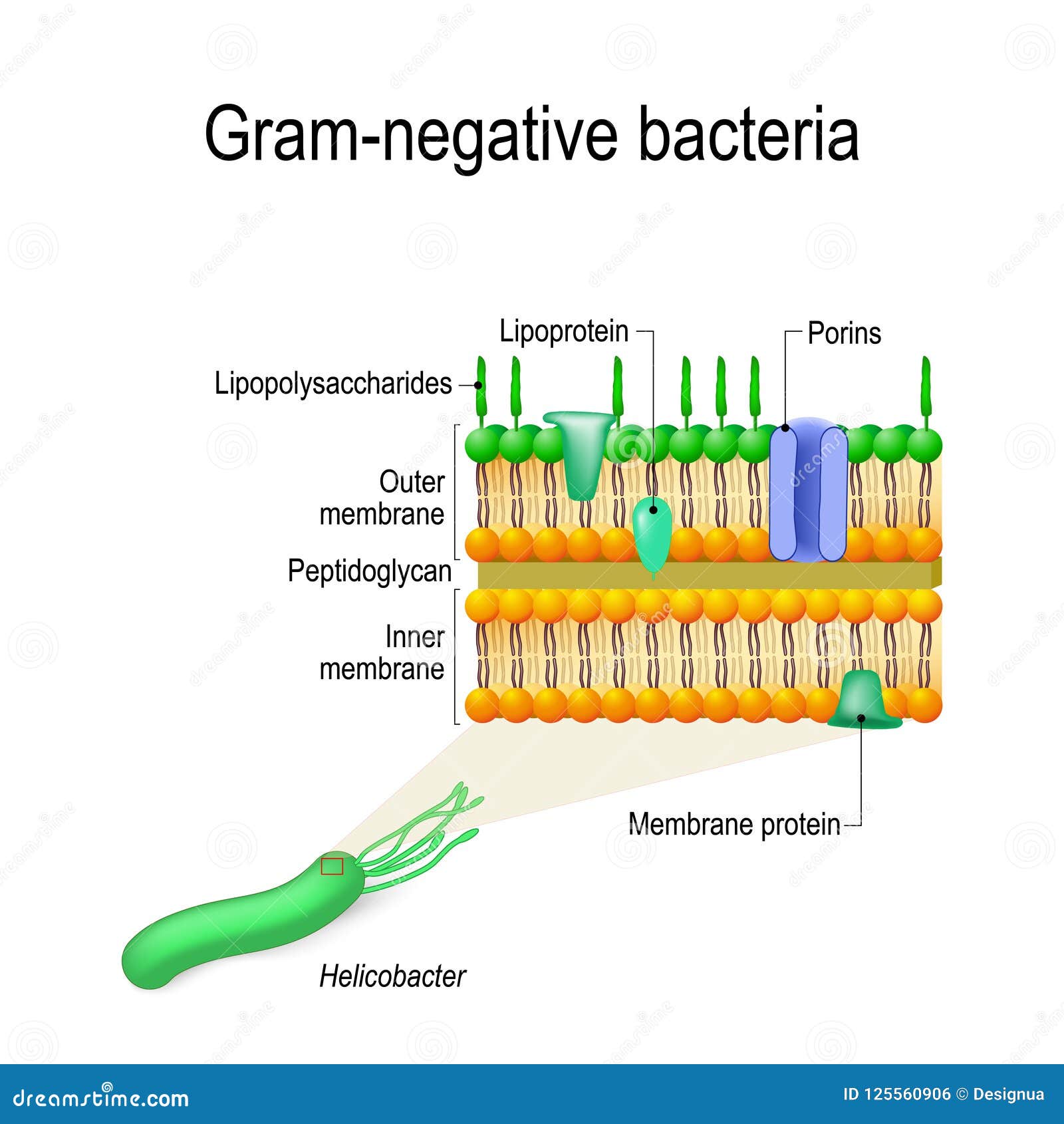
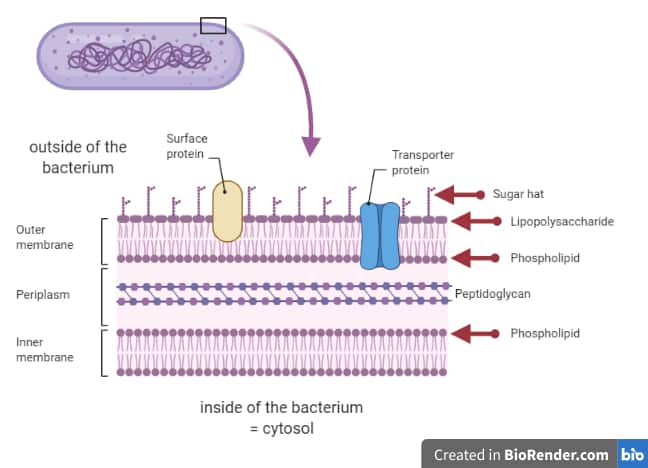
Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. These findings overturn the prevailing dogma that the cell wall is the dominant mechanical element within Gram-negative bacteria, instead demonstrating that the outer membrane can be stiffer than the cell wall, and that mechanical loads are often balanced between these structures. Both lipopolysaccharides and proteins contributed to the stiffness of the outer membrane. Compromising the outer membrane, either chemically or genetically, greatly increased deformation of the cell envelope in response to stretching, bending and indentation forces, and induced increased levels of cell lysis upon mechanical perturbation and during L-form proliferation. Here we show that the stiffness and strength of Escherichia coli cells are largely due to the outer membrane. It is widely believed that the covalently cross-linked cell wall underpins the mechanical properties of the envelope 4, 5. The envelope is a selective chemical barrier 1 that defines cell shape 2 and allows the cell to sustain large mechanical loads such as turgor pressure 3. Gram-negative bacteria possess a complex cell envelope that consists of a plasma membrane, a peptidoglycan cell wall and an outer membrane.


 0 kommentar(er)
0 kommentar(er)
